- About IVT |
- Write for Us |
- Advertise |
- Partnerships |
- Careers |
- Contact
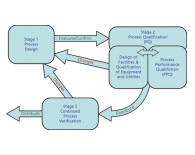
Process Validation
By Adeana Dorsey, David Fetterolf Jan 6, 2013 7:02 pm EST
Process validation involves data collection and evaluation utilizing a lifecycle management approach from design through production based on scientific evidence that a process is consistently capable of delivering quality product. The regulatory expectation of process validation has shifted the meaning of Process Validation to encompass development through continued process monitoring. Therefore, from this point forward, the term process validation used in this article will mean, “documented evidence that the process, operated within established parameters, can perform effectively and reproducibly to produce a medicinal product meeting its predetermined specifications and quality attributes."
The fundamental principle of process validation is to ensure products are manufactured in accordance with the intended use, quality, safety, and efficacy are designed or built into the product, quality cannot be tested or inspected into the final product, and each step in a manufacturing process is controlled to assure that the finished product meets all design characteristics and quality attributed including specifications. This guidance will provide a pathway for navigating the major regulatory process qualification requirements and discuss current regulatory submission feedback to meet compliance expectations.
This content is only available to IVT members. Get help maintaining your knowledge in Validation and Compliance best practices. Read More!
If you are already a member and you do not have access to this article, upgrade your membership. Need help? Read our FAQs.
Item Subjects:

