- About IVT |
- Write for Us |
- Advertise |
- Partnerships |
- Careers |
- Contact
Process Validation Toolkit
A collection of IVT's knowledge of process validation, this toolkit is composed of two years of Validation Week presentations, the Process Validation Lifecycle e-Book Series, a recorded validation seminar, a sampling instruction and a process validation whitepaper, and two validation master plans. These materials will guide process validation practitioners in effectively implementing a lifecycle approach to process validation and, thereby, achieving compliance with global regulatory agencies.
Products in this Toolkit
Process Validation | Validation Master Plan | December 17, 2012 - 4:31 am EST

The Site Validation Master Plan will help you lay out the validation program for your company. It incorporates the validation activities related to your company's manufacturing facility for the manufacturing, storage and distribution of your products.Download the Site Validation Master Plan Download the accompanying presentation "Developing a Validation Master Plan (VMP) that Survives the Life Cycle," presented at IVT's Annual Validation Week EU, Amsterdam, Netherlands, March 2011.
Process Validation, Regulatory & Industry Guidance | Regulatory & Industry Guidance | January 6, 2013 - 7:02 pm EST
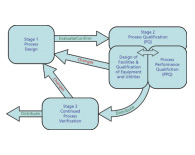
The fundamental principle of process validation is to ensure products are manufactured in accordance with the intended use, quality, safety, and efficacy are designed or built into the product, quality cannot be tested or inspected into the final product, and each step in a manufacturing process is controlled to assure that the finished product meets all design characteristics and quality attributed including specifications. This guidance will provide a pathway for navigating the major regulatory process qualification requirements and discuss current regulatory submission feedback to meet compliance...
GMP - Qualification, Process Validation | Special Edition | January 13, 2013 - 2:21 pm EST

This is Part II to IVT's three-part special edition series on process validation. This special edition is a collection of articles and papers from the Journal of Validation Technology and the Journal of GXP Compliance and focuses on equipment qualification, process qualification documentation, quality risk management, and compliant sampling. Introduction to FDA Stage 2: Process Qualification The testing performed in this process validation stage confirms that the process design is capable of manufacturing drug product at commercial scale in accordance with the process...
Design and Development, Process Validation | Special Edition | January 13, 2013 - 3:04 pm EST

This special edition is a collection of articles and papers from the Journal of Validation Technology and the Journal of GXP Compliance that expound such topics as knowledge building with probability distributions, a step-by-step process for design-of-experiment, quality-by-design, and hypothesis testing, this e-Book will firmly establish the scientific foundations of a manufacturing process.Table of Contents
Post Monitoring, Process Validation | Special Edition | January 13, 2013 - 4:11 pm EST

This is Part III of IVT's special edition series on process validation. A collection of papers and articles from the Journal of Validation Technology and the Journal of GXP Compliance, this e-Book covers controlling and reducing variation, revalidation, managing analytical error, and the process nonconformity concept Introduction to FDA Stage 3: Continuing Process Verification This stage may be simply described as “maintaining validation” or “maintaining the validated state.” Maintenance activities of Stage 3 should be commensurate with the risk identified for the...
Cleaning Validation, GMP - Stability Testing | White Paper | January 16, 2013 - 4:46 pm EST

Sampling is a key current good manufacturing practice (cGMP) activity that impacts nearly every activity of manufacturing pharmaceutical products. Sampling is used during the assessment of raw materials, labeling, and components prior to release, validation of equipment, processes, systems, and products; products during production; finished products prior to release; products during stability studies; and data before, during, and after productionThe appropriate knowledge and application of cGMP requirements for sampling is critical to the development of a scientifically sound quality...
Protocols & SOPs, GMP - Compliance & Regulations | Master Validation Plans | November 17, 2017 - 1:52 pm EST

The attached comprises three documents providing fundamental aspects of the VMP. These include an overview description of VMP and applications implemented by various organizations, an actual VMP form that would be approved by relevant groups in the organization, and an SOP providing directions to create the VMP.Validation Master Plan (VMP), Part 1. Differences in Terminology, Content, and Applications provides basic considerations associated with the topic of the Validation Master Plan (VMP). This discussion identifies four different VMP types commonly used in pharma and related...
Item Subjects:
Package Components: Site Process Validation Master Plan , Process Validation , Process Qualification , Process Design , Continuing Process Verification , A Pocket Guide to cGMP Sampling , Master Validation Plan SOP and Approval Template

